Neural Frame Metrics
Summary:
The Stanford Cancer Registry data is integrated with the California Cancer Registry and updated monthly, encompassing approximately 800 variables related to various cancer. As of January 2022, the KACI® application from NeuralFrame was approved for abstracting and transmitting diagnosed cases, with Stanford adopting it in 2023. Neural Frame connects to EPIC to extract relevant data, including pathology notes.
Within Neural Frame, Class of Case (CoC) divides cases into two groups: cancer cases are categorized as Analytic (reportable with complete data) or Non-analytic (not reportable by law, with complete data only from 2022 onward).
Analytic: Analytic cases include patients with initial diagnoses and full or partial treatments at Stanford. Analytic cases (codes 00-22) are those that are required by CoC to be abstracted because of the program’s primary responsibility in managing the cancer. Analytic cases are grouped according to the location of diagnosis and treatment.
Non-analytic: Non-analytic cases include recurrences, consults only, and in-transit patients. Non-analytic cases (codes 30-49 and 99) can be abstracted by the facility to fulfill central registry requirements or upon request from the facility’s cancer program. The grouping of non-analytic cases is based on the reason for their classification, whether they received care at the facility or were abstracted for other reasons.
📊 Data Volume
- Patients Count: 200,715
- Patients with Completed Cases: 139,046
🧬 Data Components
- Outcome
- Diagnoses
- Treatment
- Miscellaneous
Thoracic Cancer Cohort
Thoracic cancer patients are identified based on their primary site descriptions in the Neural Frame diagnoses data, which include diagnoses of lung, bronchus, or thymus cancer. The thoracic cancer cohort includes patients diagnosed with primary malignancies located in the thoracic cavity, as defined by the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) primary site codes. Specifically, the cohort is defined by the following ICD-O-3 topography codes:
- C34.0 – Main bronchus
- C34.1 – Upper lobe, lung
- C34.2 – Middle lobe, lung
- C34.3 – Lower lobe, lung
- C34.8 – Overlapping lesion of lung
- C34.9 – Lung, not otherwise specified (NOS)
- C37.9 – Thymus
Multiple Primary
Within the thoracic cancer cohort, a subset of patients presents with multiple primary thoracic malignancies. This designation refers to the presence of two or more histologically distinct malignant tumors arising independently within the thoracic cavity, rather than as metastases from a single primary site. These neoplasms may occur synchronously, identified concurrently or within a short time interval, or metachronously, developing at distinct time points.
Thoracic Cancer Metrics
This visualization summarizes the characteristics of thoracic cancer cases by:
- Cases, Diagnoses, Staging
- Treatment
- Social History
- Follow up Outcome
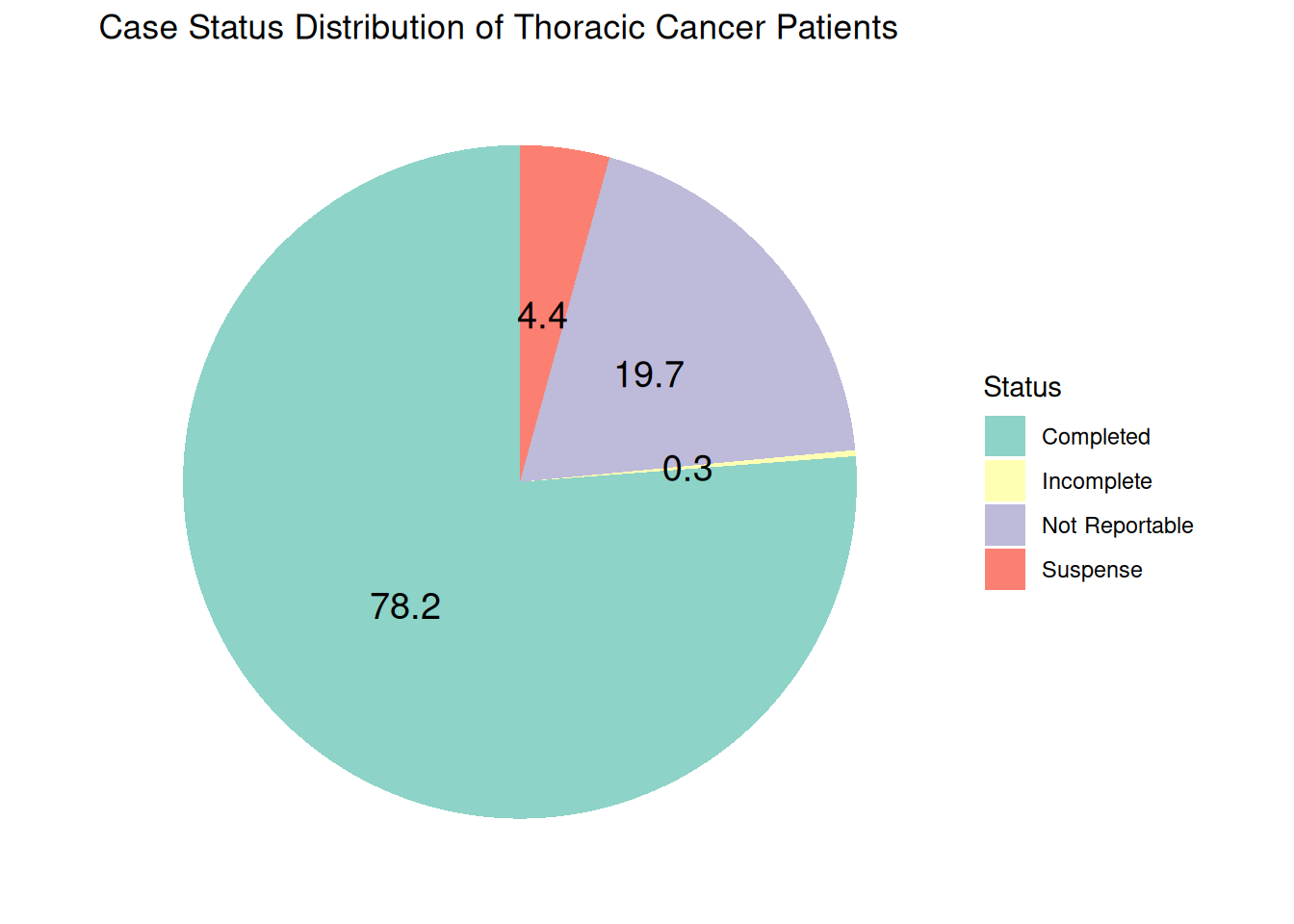
Stanford Cancer Registry Case Status
The Stanford Cancer Registry tracks all cancer cases diagnosed or treated at Stanford. Each case is assigned a case status to reflect its reporting stage. The primary categories include:
Completed: Reportable cases that have been fully abstracted and passed all edit checks. These cases are transmitted to the state registry.
Not Reportable: Cases that do not meet state reporting requirements or have already been abstracted and submitted.
Incomplete/Suspense: Reportable cases that have been abstracted but cannot yet be transmitted due to missing treatment information or unresolved edit errors. These may result from version changes, coding updates, or other registry requirements.
The case status ensures accurate tracking and timely reporting of cancer data to meet regulatory and research standards.
As of May 2025, there are 13,598 unique thoracic patients with 78.2 % completed cases.
Patient Summary by Diagnosis Year
The following distribution summarizes the number of diagnosed thoracic patients over time.
Histology Distribution & ICDO3 Codes
Thoracic cancer encompasses a diverse group of histologic subtypes, with Non-Small Cell Lung Cancer (NSCLC) representing the majority of cases and Small Cell Lung Cancer (SCLC).
Thoracic cancer is categorized into detailed histologic groups using ICD-O-3 codes and then further grouped into four overarching categories for simplified analysis. The main histology groups include:
Non-Small Cell Lung Carcinoma (NSCLC): This group encompasses the majority of lung cancer and includes subtypes such as:
- Adenocarcinoma (8140–8147)
- Squamous Cell Carcinoma (8070–8080)
- Large Cell Carcinoma (8010–8015)
- Non-Small Cell Carcinoma NOS (8046)
Small Cell Lung Carcinoma (SCLC): Represented by ICD-O-3 codes 8041–8045, this group is characterized by more aggressive, rapidly progressing tumors.
Other Specified Histologies: These include a range of less common thoracic cancer such as:
- Carcinoid Tumors (8240–8249)
- Sarcoma (8800–8806)
- Mesothelioma (9050–9053)
- Acinar Cell Carcinoma (8550–8551)
- Bronchiolo-alveolar Carcinoma (8250–8257)
- Neoplasms (8000–8005)
- Papillary Adenocarcinoma (8260–8265)
- Papillary Carcinoma (8050–8052)
Other/Unspecified Histologies: Any histology not falling into the categories above.
Thoracic Cancer Histology Distribution
From 10,634 thoracic patients with completed cases, the majority 7,420 were diagnosed with Non-Small Cell Lung Carcinoma (NSCLC).
| Histology | N(%) of Patients |
|---|---|
| NSCLC | 7,420 (69.8%) |
| Other Specified | 1,835 (17.3%) |
| Other Unspecified | 1,118 (10.5%) |
| SCLC | 551 (5.2%) |
| Note: Patients could have multiple histologies | |
Histology Type Summary
The following horizontal bar chart presents the hierarchical distribution of thoracic cancer cases, showing both major categories (NSCLC, SCLC, Other Specified, and Other Unspecified) and their corresponding histological subtypes.
- Click legend items to show/hide specific main categories
- Hover over bars to see detailed information
- Double-click legend items to isolate a single category o to restore all categories
Distribution of Thoracic Cancer Cases by Histology Types
Table Summary of Histology Groups
All the ICDO3 code and histology subgrouping are listed in the following table:
Cancer Staging
Cancer staging is categorized as:
- Stage 0: Carcinoma in situ (neoplastic cells confined to the epithelium without invasion) with no evidence of invasion beyond the basement membrane.
- Occult: A malignancy is confirmed by cytology or metastatic presentation without a known primary, meaning that the primary tumor is not clinically or radiologically identifiable.
- Stage I: A localized tumor confined to the organ of origin, with no regional lymph node involvement or distant metastasis.
- Stage II: A tumor of larger size or with limited regional extension that may involve adjacent structures or limited regional lymph nodes.
- Stage III: Locally advanced disease characterized by involvement of regional lymph nodes and/or extension to nearby tissues.
- Stage IV: Distant metastatic disease where the tumor has spread to distant organs or non-regional lymph nodes.
Summary of Staging of Thoracic Cancer Patients
The following table summarizes the staging status of thoracic patients with completed cases.
| Staging Status | Patient Count | Percentage (%) |
|---|---|---|
| Stage IV: Distant metastatic disease | 4,351 | 41 |
| Stage I: Localized tumor confined to the organ of origin | 2,785 | 26 |
| Stage III: Locally advanced disease | 1,796 | 17 |
| Unknown | 1,023 | 10 |
| Stage II: Larger tumor size or limited regional extension | 782 | 7 |
| Occult: Malignancy confirmed by cytology or metastatic presentation without known primary | 48 | 0 |
| Stage 0: Carcinoma in situ | 37 | 0 |
The following table summarizes the distribution of thoracic cancer treatment types including chemotherapy, radiation therapy, and surgery across the patient cohort with completed cases.
| Treatment | Patient Count | Percentage |
|---|---|---|
| Chemotherapy | 5,252 | 49 |
| Surgery | 4,643 | 44 |
| Radiation Therapy | 4,243 | 40 |
The following table summarizes the distribution of thoracic cancer recurrence status with completed cases.
| Recurrence Type Category | Patient Count |
|---|---|
| Never been disease-free | 6,126 |
| Disease-free after treatment and has not had a recurrence | 4,202 |
| Distant recurrence | 427 |
| It is unknown whether the disease has recurred or if the patient was ever disease-free. | 304 |
| Local recurrence | 248 |
| Disease has recurred, but the type of recurrence is unknown. | 189 |
| Regional recurrence | 97 |
From 13,598 unique thoracic cancer patients, the majority didn’t have any records of smoking status and alcohol use in Neural Frame.
Smoking Status (Neural Frame)
| Description | Patient Count |
|---|---|
| Not Available | 13,435 |
| Never used | 98 |
| Previous use | 72 |
| Cigarette smoker, current | 29 |
| Unknown | 3 |
| Note: sum of sungroups exceeds the denominator due to multiple responses by patients | |
Smoking Status (OMOP)
The information regarding the most recent smoking status of patients with thoracic cancer was generated by querying the OMOP database. For patients diagnosed with lung, bronchus, or thymus cancer, relevant patient records were linked to their smoking status in OMOP observation table by filtering out the smoking concept ID (43054909), which included multiple entries over time. By ranking these entries by date, the analysis focused on retrieving only the most recent smoking status for each patient as follow.
| Description | Patient Count |
|---|---|
| Former | 6,088 |
| Never | 4,293 |
| Every Day | 674 |
| Never Assessed | 316 |
| Some Days | 116 |
| Passive Smoke Exposure - Never Smoker | 29 |
| Unknown | 27 |
| Light Smoker | 18 |
| Heavy Smoker | 12 |
| Smoker, Current Status Unknown | 8 |
| Note: 2,017 patients didn't have smoking concept ID in the observation table | |
Alcohol Use (Neural Frame)
| Description | Patient Count |
|---|---|
| Not Available | 13,517 |
| No history of alcohol use | 61 |
| Current use of alcohol | 31 |
| Past history of alcohol use, does not currently use | 10 |
| Alcohol usage unknown | 3 |
| Note: sum of sungroups exceeds the denominator due to multiple responses by patients | |